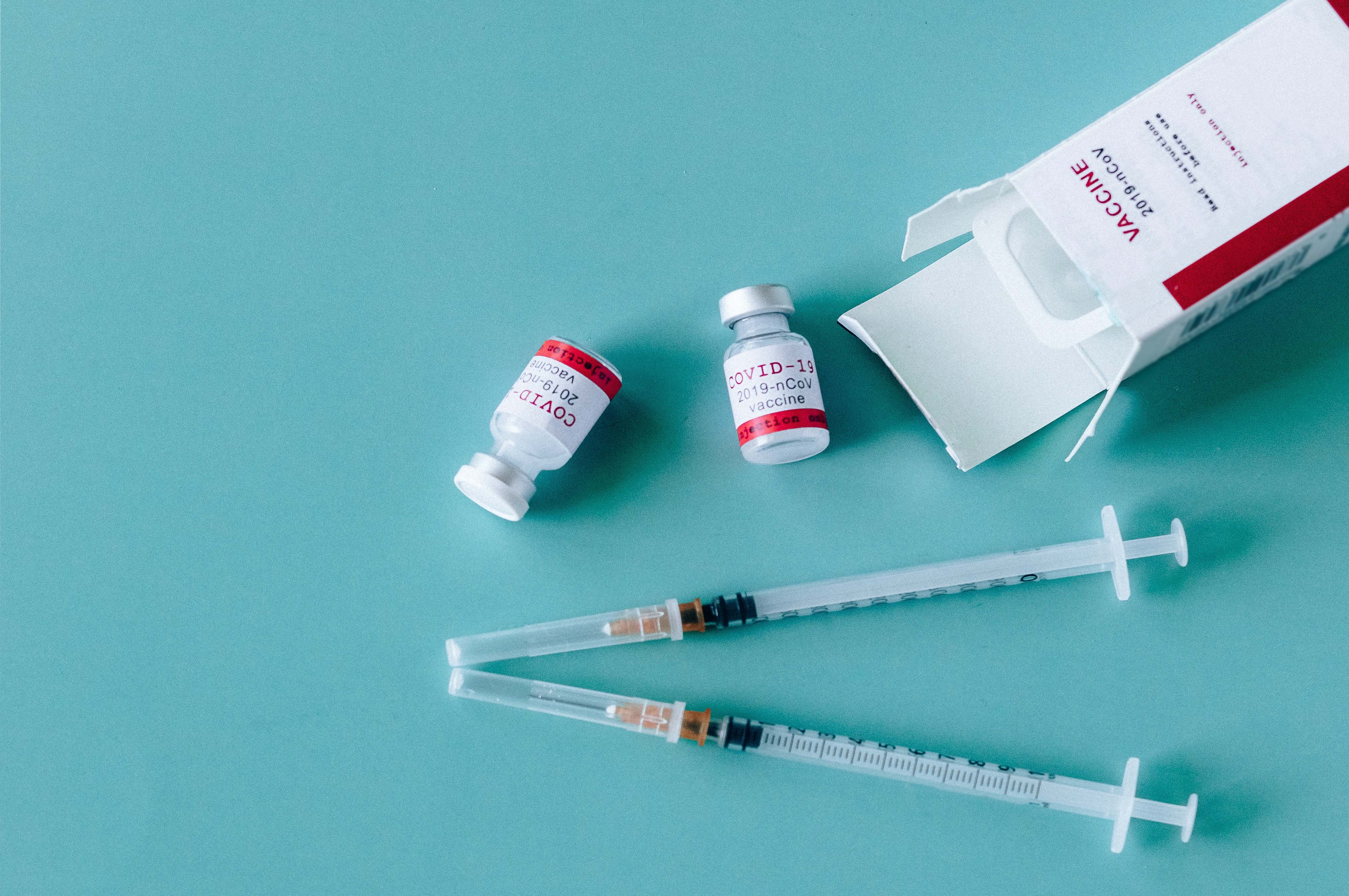
Which of the two vaccines should I get? The simple answer is — it doesn't matter. Both have been tested in large numbers of older adults and have been found to be safe and effective.
At the time of this publication, two vaccines are approved for Emergency Use Authorization. Pfizer-BioNTech's mRNA vaccine for those 16 years of age and older. Two 0.3 ml doses of the Pfizer vaccine given 21 days apart are 95% effective in preventing severe COVID-19 disease. The second is the Moderna mRNA vaccine for those 18 years of age and older. It requires two 0.5ml doses given 28 days apart, and it is 94.1% effective in preventing severe COVID-19 disease. Scientific evidence supports the need for the two doses to reach maximum effect.
These two vaccines are not interchangeable. If your first vaccine is a Pfizer vaccine, the second one must be the same. This is also true for the Moderna vaccine series. CDC has indicated that people may receive the second dose of either vaccine up to 4 days before the scheduled second dose or up to six weeks after that dose is scheduled to be given, but the 21- and 28- day dosing intervals are still recommended.
Clinical trials for both vaccines included older adults and have been shown to be equally safe and effective in that population. Of the ~40,000 participants in the Pfizer trial, ~7,600 were 65 and older. Of the ~30,000 participants in the Moderna trial, ~7,700 were 65 and older, and ~4,400 of those were 75 and older. For now and the foreseeable future, people will not choose which vaccine they receive. They will receive whatever vaccine is being dispensed at the site where they make an appointment. Since both are equally safe and effective, the important message is to get vaccinated as soon as possible.
Johnson and Johnson is expected to apply to the FDA within weeks for Emergency Use Authorization of its vaccine, which does not use mRNA technology like Pfizer and Moderna. The Johnson and Johnson vaccine uses a modified adenovirus that will build mRNA and provoke the immune system to develop antibodies. Their clinical trial was conducted globally and included nearly 44,000 people. It demonstrated prevention of the disease in the US at 72%, Latin America at 66%, and South Africa at 57%, with prevention of severe forms of COVID-19 at 85%.
Although the effectiveness isn't as high as the Pfizer and Moderna vaccines, there are advantages. It requires only one dose and does not need to be stored at extreme temperatures, as do the Moderna and Pfizer vaccines.
New variants of COVID-19 are emerging similarly to the way new flu variants appear year to year. Although the vaccines available today seem to be effective with current COVID-19 variants, it is reasonable to think we may need a booster shot at least yearly to be fully protected against new variants. Moderna is already working on a booster, and the good news is that as we now have the vaccines, modifications are much easier.
----

 Donation
Donation